The Gut-Brain Axis: A Definitive Guide to Enhancing Mental Health Through Gut Health
Poor Mental health is a pressing global concern impacting millions worldwide, encompassing a spectrum of related conditions. In this article, we embark on a journey to unravel the multifaceted connections between the gut and mental health, delving into the pivotal role of diet, lifestyle factors, electromagnetic fields (EMFs), and sleep patterns in influencing these interrelated domains. By examining the latest research and insights, we aim to shed light on the dynamic interplay between gut health and mental wellness, offering insights that may pave the way for better understanding and improved quality of life for affected individuals.
Back to basics: The Gut-Brain Connection
Emerging research has shown the profound influence of the gut microbiome on neurotransmitter and hormone production, which is crucial for mood regulation. Imbalances in gut bacteria correlate with conditions like depression, anxiety, and ADHD, proving the necessity of a comprehensive approach to mental health addressing gut health.
Even though it has been known for millennia, the scientific community has been captivated by the intricate interplay between the gut and the brain in recent years. This revelation has shattered conventional beliefs about mental health, unveiling a dynamic relationship that extends far beyond the confines of the skull. At the heart of this revelation lies the gut microbiome, a bustling ecosystem of trillions of microorganisms that call our digestive tract home.
As researchers delve deeper into the mysteries of the gut-brain axis, they uncover a web of connections that defy traditional boundaries. It's a symbiotic relationship, where the gut microbiome acts as a silent conductor orchestrating a symphony of biochemical signals reverberating throughout the body and mind. Among these signals are neurotransmitters and hormones, the chemical messengers that govern our moods, emotions, and cognitive functions.
The revelation that our gut bacteria possess the power to manufacture neurotransmitters and hormones has profound implications for our understanding of mental health. It suggests that the key to unlocking the mysteries of conditions like depression, anxiety, and ADHD may lie not only in the recesses of the mind but also within the depths of our digestive system. Indeed, emerging research has begun to unravel the intricate connections between gut health and mental wellness, painting a compelling picture of the gut as a central player in the drama of the mind.
Imbalances in gut bacteria, researchers have found, are not merely coincidental companions to mental health disorders but active participants in their genesis and progression. The discovery that individuals suffering from depression, anxiety, and ADHD often exhibit disruptions in their gut microbiome has ignited a fervent pursuit of solutions that transcend traditional pharmaceutical interventions. It's a call to arms for a holistic approach to mental health that acknowledges gut health's integral role in shaping our psychological well-being.
As we embark on this journey to explore the gut-brain connection, we are confronted with a tantalising prospect: the promise of a new era in mental health care. It's an era where dietary interventions, lifestyle modifications, and innovative therapies hold the potential to revolutionise the way we understand and treat mental health disorders. But to unlock this potential, we must first unravel the mysteries of the gut-brain axis and embrace the profound implications of its discoveries.
The Gut-Brain Relationship: The Role of Gut Microbiota in Mental Health
1. Neurotransmitter Regulation:
Research affirms the significant role of gut microbiota in modulating neurotransmitter production, immune responses, and inflammation, influencing conditions like depression, anxiety, and schizophrenia. Thus, fostering a healthy gut microbiome through dietary and lifestyle interventions is essential in mental health management.
The gut microbiota actively communicates with the brain through the gut-brain axis (the vagus nerve), influencing neurotransmitter production. For instance, certain bacteria in the gut produce neurotransmitters like serotonin and GABA, which play key roles in regulating mood. Imbalances in these neurotransmitters have been linked to various mental health disorders.
Despite traditionally being associated with brain function, these neurotransmitters are primarily synthesised within the gut. Although they can’t cross the blood-brain barrier, their production in the gut significantly influences their levels within the brain.
Additionally, the gut microbiome influences the immune system and inflammation levels, further impacting mental well-being. The connection between the gut microbiome and mental health goes beyond digestion. Gut bacteria communicate with the immune system. When this balance is disturbed, it can trigger inflammation (either as a result of dysbiosis or food intolerances and allergies). Gut inflammation is linked to conditions like depression and anxiety. Therefore, a healthy gut is essential to regulate the immune system and reduce inflammation, which can improve mental well-being.
Understanding the Gut-Brain Axis
The gut-brain axis constitutes a bidirectional communication system between the central nervous system and the gastrointestinal tract. Disruptions in this axis, often induced by stress and inflammation, can perturb gut bacteria composition and heighten the risk of mental health conditions like depression and anxiety.
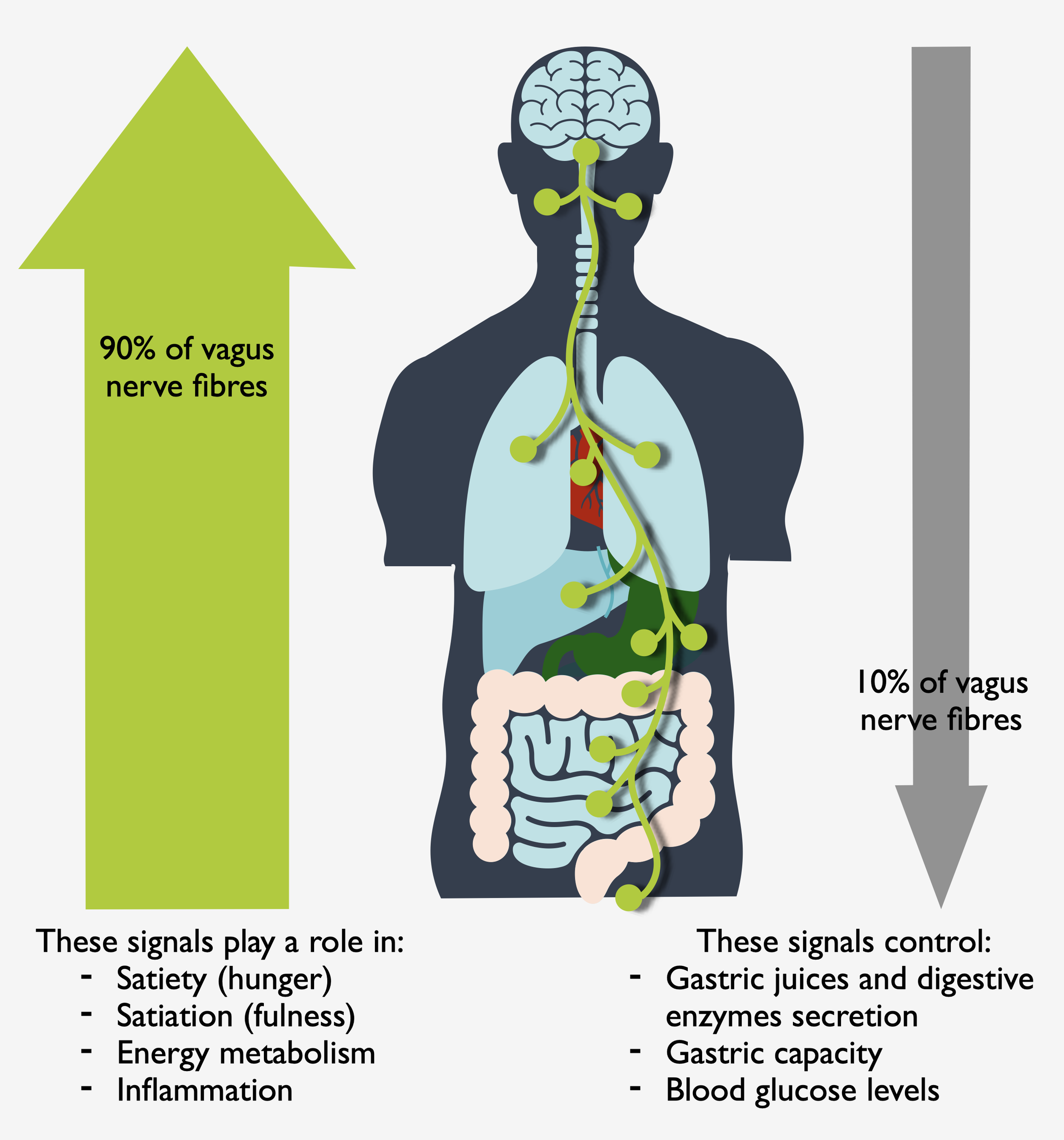
The gut-brain axis and the vagus nerve
Stress, inflammation, and other environmental factors can disrupt the delicate balance of the gut-brain axis, leading to gut microbiota composition alterations and functionality. This disruption can contribute to the development and exacerbation of mental health disorders. Moreover, bidirectional communication means that signals from the gut can influence brain function and vice versa, highlighting the interconnectedness of gut health and mental well-being.
Factors Affecting the Gut-Brain Relationship
Stress-induced gut dysbiosis, antibiotic usage, and certain medications like antacids and oral contraceptives profoundly influence gut health and may exacerbate mental health conditions. Conversely, probiotics offer promise in restoring microbial balance and ameliorating mental health symptoms.
Indeed, chronic stress can disrupt the gut microbiota composition, leading to dysbiosis and increased intestinal permeability. This allows harmful bacteria, their byproducts, allergens, and toxins to enter the bloodstream, triggering inflammation and potentially exacerbating mental health conditions. Antibiotics, while necessary for treating infections, can also indiscriminately destroy beneficial gut bacteria, further contributing to dysbiosis. It is, therefore, essential to support the gut during the entire course of the treatment and some time after.
Gut's Impact on Mental Health
— Depression
Research has demonstrated the correlation between gut microbiome alterations and depressive symptoms. Interventions like probiotic supplementation and stress reduction techniques show promise in mitigating depression.
Studies have demonstrated that individuals with depression often exhibit altered gut microbiota composition compared to healthy individuals. These imbalances may contribute to systemic inflammation and affect neurotransmitter production, influencing mood.
looking at certain probiotics, such as psychobiotics, have shown potential in modulating the gut microbiota and alleviating depressive symptoms by reducing inflammation and restoring neurotransmitter balance. To some extent, prebiotic fibres can be classified as psychobiotics by directly affecting the integrity of the gut milieu and the release of bacterial metabolites.
Additionally, stress reduction techniques like mindfulness meditation and exercise can positively impact gut health and mental well-being by mitigating stress-induced dysbiosis.
Depression is often associated with dysregulation of neurotransmitters, which are chemical messengers that transmit signals between nerve cells in the brain. Here's how depression is involved in neurotransmitter dysregulation:
Serotonin:
Serotonin is a neurotransmitter known for its role in regulating mood, appetite, and sleep. In depression, there may be decreased levels of serotonin or impaired serotonin signalling. This can lead to feelings of sadness, low mood, and changes in appetite and sleep patterns.
Dopamine:
Dopamine is involved in the brain's reward system and plays a role in motivation, pleasure, and reinforcement of rewarding behaviours. Dysregulation of dopamine levels or signalling has been implicated in depression, contributing to symptoms such as anhedonia (loss of interest or pleasure) and lack of motivation.
Norepinephrine:
Norepinephrine (noradrenaline), is involved in the body's "fight or flight" response and helps regulate mood and stress. Dysregulation of norepinephrine has been linked to depressive symptoms such as fatigue, lack of energy, and decreased concentration.
GABA (Gamma-Aminobutyric Acid):
GABA is the primary inhibitory neurotransmitter in the central nervous system and helps regulate anxiety and stress response. Dysregulation of GABAergic neurotransmission has been implicated in depression, contributing to symptoms such as anxiety and restlessness.
Depression can dysregulate neurotransmitters through various mechanisms, including alterations in synthesis, release, reuptake, and receptor sensitivity:
Synthesis:
Depression may interfere with the synthesis of neurotransmitters, reducing their production. For example, disruptions in the availability of precursor molecules or changes in enzyme activity involved in neurotransmitter synthesis can lead to decreased levels of neurotransmitters such as serotonin, dopamine, and norepinephrine.
Release:
Depressive disorders can impact the release of neurotransmitters from nerve cells into the synaptic cleft, where they can interact with receptors on neighbouring neurons. Dysregulation of release mechanisms, including impaired vesicular transport or altered calcium ion channels, may result in reduced neurotransmitter release and impaired signalling between neurons.
Reuptake:
Neurotransmitter reuptake is the process by which neurotransmitters are removed from the synaptic cleft and recycled by the presynaptic neurone. Dysregulation of neurotransmitter reuptake transporters, such as the serotonin transporter (SERT) or the dopamine transporter (DAT), can lead to increased reuptake of neurotransmitters, reducing their availability in the synaptic cleft and impairing neurotransmission.
Receptor Sensitivity:
Depression can alter the sensitivity of neurotransmitter receptors on postsynaptic neurons, affecting their response to neurotransmitter binding. Changes in receptor density, affinity, or downstream signalling pathways can impact the efficacy of neurotransmitter signalling and contribute to depressive symptoms.
— Anxiety
Dysbiosis is intricately linked to anxiety disorders, with dietary modifications and stress management strategies presenting as potential interventions.
Anxiety disorders are often associated with alterations in gut microbiota composition and increased gut permeability. These changes can lead to heightened immune responses and systemic inflammation, contributing to anxiety symptoms. Dietary modifications, such as increasing fibre intake and consuming pre- and probiotic-rich foods, can promote the growth of beneficial gut bacteria and improve gut health, potentially reducing anxiety symptoms.
Again, stress management techniques like relaxation exercises and cognitive-behavioural therapy can help mitigate stress-induced gut dysbiosis and alleviate anxiety.
Pathways involved in neurotransmitter dysregulation in anxiety:
GABA-Glutamate Pathway:
The balance between the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) and the excitatory neurotransmitter glutamate is crucial for regulating anxiety. GABA inhibits neural activity, promoting relaxation and reducing anxiety, while glutamate stimulates neural activity, contributing to anxiety. Dysregulation of the GABA-glutamate pathway, such as decreased GABAergic activity or increased glutamatergic activity, can lead to heightened anxiety levels.
Serotonin Pathway:
Serotonin, also known as 5-hydroxytryptamine (5-HT), is involved in mood regulation and emotional processing, making it relevant to anxiety disorders. Dysregulation of the serotonin pathway, particularly reduced serotonin levels or impaired serotonin receptor function, is associated with increased anxiety.
Serotonin modulates neural circuits involved in fear processing and anxiety responses, and alterations in serotonin transmission can disrupt these circuits, contributing to anxiety disorders.
Norepinephrine Pathway:
Norepinephrine (noradrenaline), is involved in the body's stress response and arousal regulation. Dysregulation of the norepinephrine pathway, characterised by increased noradrenergic activity, is implicated in anxiety disorders.
Norepinephrine acts on adrenergic receptors in the brain to modulate arousal, attention, and vigilance, and abnormalities in norepinephrine transmission can lead to heightened anxiety and hyperarousal states.
Hypothalamic-Pituitary-Adrenal (HPA) Axis:
The HPA axis is a key neuroendocrine system involved in the body's response to stress. Dysregulation of the HPA axis, characterised by hyperactivity or impaired feedback mechanisms, is associated with anxiety disorders. Chronic stress or trauma can dysregulate the HPA axis, leading to excessive cortisol release and alterations in stress response systems, which contribute to anxiety symptoms.
Neuropeptide Pathways:
Neuropeptides such as corticotropin-releasing hormone (CRH), neuropeptide Y (NPY), and substance P are involved in modulating anxiety-related behaviours and stress responses. Dysregulation of neuropeptide pathways, characterized by aberrant neuropeptide release or receptor function, can contribute to anxiety disorders. CRH, for example, plays a central role in initiating the body's stress response, and abnormalities in CRH signalling have been implicated in anxiety disorders.
Pathways involved in neurotransmitter dysregulation in anxiety:
Anxiety can dysregulate neurotransmitters through similar mechanisms as depression, contributing to the manifestation of anxiety symptoms:
Synthesis:
Anxiety disorders may disrupt the synthesis of neurotransmitters, leading to altered levels of key signalling molecules in the brain. For example, dysregulation of enzymes involved in neurotransmitter synthesis, such as tryptophan hydroxylase for serotonin or tyrosine hydroxylase for dopamine, can result in imbalances in neurotransmitter production.
Release:
Dysregulation of neurotransmitter release mechanisms can also contribute to anxiety. Heightened neuronal activity or alterations in calcium ion channels can lead to excessive neurotransmitter release, resulting in hyperexcitability of neural circuits associated with anxiety-related behaviours.
Reuptake:
Changes in neurotransmitter reuptake transporters, in the same way they are found in depression, can impact anxiety symptoms. Dysfunctional reuptake transporters may lead to impaired clearance of neurotransmitters from the synaptic cleft, prolonging their effects and disrupting neural signalling.
Receptor Sensitivity:
Anxiety disorders can alter the sensitivity of neurotransmitter receptors in the brain. Changes in receptor density, affinity, or intracellular signalling pathways can affect the responsiveness of postsynaptic neurons to neurotransmitter binding, influencing the transmission of anxiety-related signals.
— Schizophrenia
While genetic factors predominate in schizophrenia, growing evidence implicates gut dysbiosis and chronic inflammation in its pathogenesis, warranting further exploration.
Schizophrenia is a complex mental disorder with multifactorial origins, including genetic predisposition and environmental factors. Recent research suggests that alterations in the gut microbiota composition and increased intestinal permeability may contribute to immune system dysregulation and chronic inflammation, which could exacerbate schizophrenia symptoms. Further investigation into the gut-brain axis and its role in schizophrenia may uncover novel therapeutic targets for managing the disorder.
— Autism
The interplay between gut microbiota and autism spectrum disorder suggests potential therapeutic interventions targeting the gut-brain axis.
Autism spectrum disorder (ASD) is characterised by communication deficits, repetitive behaviours, and social impairments. Emerging evidence indicates a correlation between gut microbiota alterations and ASD-related behaviours, suggesting a potential role of the gut-brain axis in the disorder's pathogenesis.
A sugar-based diet seems to be responsible for the severity of symptoms.
Modulating gut microbiota composition through dietary interventions, increased dietary fibre intake, probiotic supplementation, and other gut-healthy interventions may offer avenues for managing ASD symptoms and improving the overall quality of life for individuals with ASD.
Autism spectrum disorder (ASD) is associated with alterations in neurotransmitter function, which contribute to the characteristic symptoms of the condition:
Serotonin:
Dysregulation of the serotonin system is implicated in autism. Serotonin is involved in various brain functions, including mood regulation, social behaviour, and sensory processing, all of which are affected in individuals with ASD. Changes in serotonin synthesis, release, or receptor sensitivity may disrupt normal brain development and contribute to the behavioural and cognitive features of autism.
GABA:
GABA is the main inhibitory neurotransmitter in the brain and plays a crucial role in regulating neuronal excitability. Altered GABAergic neurotransmission has been observed in individuals with autism and may contribute to sensory processing difficulties, repetitive behaviours, and impaired social interaction. Dysregulation of GABAergic signalling can disrupt the balance between excitation and inhibition in the brain, leading to abnormal neural activity and behavioural abnormalities.
Glutamate:
Glutamate is the primary excitatory neurotransmitter in the brain and is involved in synaptic plasticity, learning, and memory. Dysregulation of glutamatergic neurotransmission has been implicated in autism, with alterations in glutamate receptor expression, function, and signalling pathways observed in individuals with ASD. Abnormalities in glutamatergic transmission may affect neuronal connectivity and synaptic communication, contributing to the cognitive and behavioural symptoms of autism.
Oxytocin:
Oxytocin is a neuropeptide involved in social bonding, trust, and emotional regulation. Dysregulation of the oxytocin system has been proposed as a potential factor in the social deficits observed in autism. Changes in oxytocin levels, receptor expression, or signalling pathways may impair social cognition and interaction in individuals with ASD.
Dopamine:
Dopamine is involved in reward processing, motivation, and motor control. Dysregulation of dopaminergic neurotransmission has been implicated in autism, particularly in relation to repetitive behaviours and restricted interests. Altered dopamine receptor expression or function may contribute to behavioural inflexibility and stereotyped movements characteristic of ASD.
Pathways involved in autism:
Serotonin Pathway:
Serotonin plays a crucial role in early brain development and synaptic plasticity. Dysregulation of the serotonin pathway, particularly abnormalities in serotonin synthesis, transporter function, or receptor expression, has been implicated in autism spectrum disorder (ASD). Altered serotonin levels or signalling during critical periods of brain development can disrupt neuronal connectivity and synaptic transmission, contributing to the pathophysiology of ASD.
Sugar-based diets, high in refined sugars and lacking essential nutrients, may exacerbate serotonin dysregulation by promoting oxidative stress and inflammation, which can further impair serotonin synthesis and signalling pathways, exacerbating autistic symptoms.
Dopamine Pathway:
Dopamine, another neurotransmitter involved in various brain functions, including reward processing, motivation, and motor control, has been linked to autism. Dysregulation of the dopamine pathway, characterised by abnormalities in dopamine synthesis, release, or receptor signalling, may contribute to the behavioural symptoms observed in ASD. Disrupted dopaminergic transmission can affect social interaction, repetitive behaviours, and sensory processing, which are core features of autism.
Sugar-based diets can influence dopamine signalling by altering dopamine receptor expression and dopamine release in the brain, potentially exacerbating behavioural abnormalities associated with autism.
Oxytocin Pathway:
Oxytocin, the "love hormone" or "bonding hormone," plays a key role in social affiliation, trust, and attachment behaviours. Dysregulation of the oxytocin pathway, involving alterations in oxytocin release, receptor expression, or oxytocinergic signalling, has been implicated in ASD. Impaired oxytocinergic function may contribute to deficits in social communication and interaction observed in individuals with autism.
Glutamate-GABA Balance:
The balance between the excitatory neurotransmitter glutamate and the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) is essential for maintaining neural circuitry and synaptic transmission in the brain. Dysregulation of the glutamate-GABA balance, characterized by altered glutamatergic or GABAergic function, has been implicated in ASD. Imbalances in excitatory and inhibitory neurotransmission can disrupt neuronal connectivity, synaptic plasticity, and information processing, contributing to the neurodevelopmental abnormalities associated with autism.
Neurodevelopmental Pathways:
Several neurodevelopmental pathways and processes, including synaptic pruning, neuronal migration, and axon guidance, are disrupted in autism. Genetic mutations or environmental factors affecting neurodevelopmental pathways can lead to structural and functional abnormalities in the brain, contributing to the core symptoms of ASD. Aberrant neural circuitry, connectivity, and cortical organisation may underlie the cognitive, behavioural, and sensory differences observed in individuals with autism.
Sugar-based diets, by promoting inflammation and impairing neurogenesis, may disrupt key neurodevelopmental processes, further exacerbating the neurobiological abnormalities associated with autism.
Factors Exacerbating Mental Health Conditions
— Diet
Diet plays a crucial role in shaping the composition and function of the gut microbiota, which in turn influences mental health outcomes. Whole foods, such as fruits, vegetables, whole grains, and fatty fish, provide essential nutrients and fibre that promote the growth of beneficial gut bacteria and reduce inflammation. Conversely, diets high in processed foods, sugar, and unhealthy fats can disrupt gut microbiota balance and exacerbate symptoms of mental health disorders. Therefore, a nutrient-dense diet is essential to support gut health and improve mental well-being.
— Lifestyle
Sedentary behaviour and unhealthy lifestyle habits, such as smoking, excessive alcohol consumption, and poor dietary choices, can negatively impact gut microbiota diversity and function. Physical activity stimulates gut motility and promotes the growth of beneficial gut bacteria, while unhealthy behaviours like smoking and excessive alcohol intake can disrupt gut microbiota balance and increase inflammation.
Therefore, adopting a physically active lifestyle and making healthy dietary choices are integral to supporting gut health and preventing the exacerbation of mental health conditions.
Remember to keep hydrated all day long, instead of waiting to feel thirsty.
— EMFs
Electromagnetic fields (EMFs) emitted by electronic devices, such as smartphones, laptops, and Wi-Fi routers, have been shown to affect gut microbiota composition and function.
Prolonged exposure to EMFs can disrupt the balance of beneficial and pathogenic gut bacteria, leading to dysbiosis and increased intestinal permeability. These changes may contribute to systemic inflammation and neuroinflammation, which have been linked to the development of mental health disorders like anxiety and depression.
Minimising exposure to EMFs and implementing protective measures, such as using EMF shielding devices and practising digital detoxification, may help mitigate the adverse effects on gut health and mental well-being.
It is also important to monitor the use of smart and connected devices in young children and to minimise the exposure of a developing body and brain to the health-damaging impact of EMFs.
— Vaccines: The Controversial Link
Vaccines stimulate the immune system to produce an immune response against specific pathogens, helping prevent infectious diseases. However, concerns have been raised about the potential side effects of vaccines on gut health and mental well-being.
Some studies suggest that certain vaccine ingredients, such as adjuvants and preservatives, such as mercury and aluminium (well-studied neurotoxins) may disrupt gut microbiota balance and contribute to immune system dysregulation. They may also cross the blood-brain barrier and inflame neurones.
Because of a lack of honesty and consensus, the evidence supporting this link is limited (and well-hidden by the Pharma empire), necessitating honest and open research to elucidate the potential effects of vaccines on gut health and mental well-being.
— Sleep
Sleep regulates various physiological processes, including immune function, hormone regulation, and gut health. Inadequate sleep duration or poor sleep quality can disrupt circadian rhythms and alter gut microbiota composition and function.
Sleep deprivation has been associated with increased gut permeability, inflammation, and dysbiosis, which may contribute to the development or exacerbation of mental health disorders. Therefore, prioritising healthy sleep habits, such as maintaining a consistent sleep schedule, creating a conducive sleep environment, and practising relaxation techniques, is essential for supporting gut health and promoting mental well-being.
Chronic lack of sleep is also associated with mood swings, irritability, forgetfulness, all of which are exacerbated by poor sleep and dysbiosis, in a self-feeding cycle.
Conclusion
The gut-brain axis is a bidirectional communication system between the gut and the brain, linking emotional and cognitive centres of the brain with peripheral intestinal functions. This axis involves various pathways, including the nervous system, immune system, and endocrine system, which facilitate communication between the gut and the brain.
One key aspect of the gut-brain axis is the influence of the gut microbiota on brain function and mental health. The gut microbiome produces neurotransmitters and metabolites that can affect mood, cognition, and behaviour. For example, certain bacteria in the gut produce gamma-aminobutyric acid (GABA) and serotonin, neurotransmitters that play essential roles in regulating mood and anxiety. Additionally, microbial metabolites such as short-chain fatty acids (SCFAs) can modulate brain function and inflammation, further impacting mental well-being.
By improving gut health through dietary interventions, prebiotics, probiotics (psychobiotics), and lifestyle modifications, individuals can positively influence the gut-brain axis and promote better mental health outcomes. Consuming a balanced nutrient-dense diet, rich in fruits and vegetables, fibre, prebiotics, and fermented foods can support the growth of beneficial gut bacteria and enhance microbial diversity. Probiotic supplements containing specific strains of bacteria may also help restore microbial balance and improve gut-brain communication.
Furthermore, reducing exposure to environmental stressors such as chronic stress, poor sleep, and dietary toxins can help maintain gut integrity and prevent dysbiosis. Strategies to manage stress and promote relaxation, such as mindfulness meditation and regular exercise, can also benefit both gut health and mental well-being.
In addition to dietary interventions and lifestyle modifications, several other modalities can be employed to enhance the gut-brain axis and improve mental health. These approaches include:
Mind-Body Practices: Techniques such as yoga, tai chi, and qigong promote relaxation, reduce stress, and enhance mind-body awareness. These practices have been shown to modulate the gut microbiota and improve mental well-being by reducing inflammation and promoting a sense of calmness.
Incorporating mindfulness-based practices, such as meditation, yoga, and tai chi, into daily life can have profound effects on mental health and the gut-brain axis. Mindfulness techniques promote present-moment awareness, reduce stress levels, and foster emotional resilience. Research suggests that mindfulness practices may also positively influence gut health by modulating the gut microbiota and reducing inflammation. By cultivating mindfulness, individuals can develop a greater sense of inner peace, clarity, and well-being, which can in turn support overall mental health and gut function.
Art Therapy: Engaging in creative activities like painting, drawing, or crafting can serve as a therapeutic outlet for individuals experiencing mental health challenges. Art therapy encourages self-expression, reduces stress, and fosters a sense of accomplishment and empowerment. While not directly targeting the gut-brain axis, the positive impact of art therapy on mental well-being can indirectly influence gut health by alleviating symptoms of depression and anxiety. Incorporating art therapy alongside dietary and lifestyle modifications may offer a multi-dimensional approach to enhancing mental health through gut health.
Nutritional Supplements: Certain nutritional supplements, such as omega-3 fatty acids, magnesium, and vitamin D, have been studied for their potential to support gut health and alleviate symptoms of depression and anxiety. These supplements may help address nutrient deficiencies and provide additional support for mental well-being.
Ensure to consume enough protein. Protein contains chemicals called amino acids, which your brain needs to produce chemicals called neurotransmitters. These help to regulate your thoughts and feelings.
Deficiencies in neurotransmitters such as serotonin, dopamine, noradrenaline, and γ-aminobutyric acid (GABA) are often associated with depression. As reported in several studies, the amino acids tryptophan, tyrosine, phenylalanine, and methionine are often helpful in treating many mood disorders including depression.
Omega–3 fatty acids, vitamin B (e.g., folate) and magnesium deficiencies have been linked to depression and other mental disrorders.
Dietary deficiencies of antioxidants and nutrients (trace elements, vitamins, and nonessential micronutrients such as polyphenols) during ageing may precipitate brain diseases, which may be due to failure for protective mechanism against free radicals.
Selenium, zinc and chromium also play a huge role in mental health and are typical cofactors in many enzymatic pathways.
Herbal Medicine: Herbal remedies like chamomile, lavender, and passionflower have been traditionally used to promote relaxation and alleviate symptoms of stress and anxiety. Herbal supplements and teas containing these botanicals may offer natural support for gut health and mental well-being.
Certain herbs, such as ashwagandha, rhodiola, and holy basil, have adaptogenic properties, meaning they help the body adapt to stress and promote balance. These herbs may modulate the stress response, enhance resilience, and support gut health by reducing inflammation and promoting microbial diversity. Additionally, herbal supplements like ginger, peppermint, and licorice root have been traditionally used to aid digestion and soothe gastrointestinal discomfort. Incorporating these natural remedies may provide additional support and promote optimal mental well-being.
Acupuncture: Acupuncture, a traditional Chinese medicine practice involving the insertion of thin needles into specific points on the body, has been shown to modulate the gut-brain axis and regulate neurotransmitter levels. This holistic approach may help alleviate symptoms of depression, anxiety, and other mental health conditions.
Psychological Therapies: Therapies such as cognitive behavioural therapy (CBT), mindfulness-based stress reduction (MBSR), and dialectical behaviour therapy (DBT) can help individuals develop coping strategies, improve emotional regulation, and enhance resilience. These approaches may complement gut-focused interventions by addressing psychological factors contributing to mental health issues.
Gut Microbiota Therapies: Emerging research suggests that interventions aimed at modulating the gut microbiota, such as faecal microbiota transplantation (FMT) and microbial-based therapies, hold promise for improving mental health outcomes. These therapies involve transplanting beneficial bacteria into the gut to restore microbial balance and support overall well-being.
Sleep Hygiene: Prioritising good sleep habits, such as maintaining a consistent sleep schedule, creating a relaxing bedtime routine, and ensuring a comfortable sleep environment, can significantly impact mental well-being. Poor sleep quality and disruptions in sleep patterns have been linked to increased symptoms of depression, anxiety, and other mental health disorders.
Stress Management: Adopting effective stress management techniques, such as deep breathing exercises, mindfulness meditation, and progressive muscle relaxation, can help reduce stress levels and promote emotional resilience. Chronic stress has detrimental effects on the gut-brain axis and may exacerbate symptoms of mental health conditions.
Social Support: Cultivating strong social connections and maintaining supportive relationships with friends, family, and community members is essential for mental health. Social support provides a buffer against stress and adversity, fosters a sense of belonging and purpose, and promotes emotional well-being.
Physical Activity: Regular exercise has numerous benefits for both physical and mental health. Engaging in physical activity releases endorphins, improves mood, reduces stress, and enhances cognitive function. Incorporating regular exercise into one's routine can positively impact the gut-brain axis and contribute to better mental health outcomes.
Consider forest bathing or gardening to help you reconnect with nature and yourself.
Hydration: Staying hydrated is crucial for optimal gut function and overall health. Drinking an adequate amount of water helps maintain proper digestion, supports nutrient absorption, and facilitates the elimination of toxins from the body. Dehydration can exacerbate digestive issues and negatively affect mood and cognitive function.
In summary, understanding and nurturing the gut-brain axis through targeted interventions can be a powerful approach to enhancing mental health. By optimising gut health, individuals can positively influence brain function, mood regulation, and overall well-being, ultimately leading to a happier and healthier life.
The intricate interplay between gut health and mental well-being requires a holistic approach encompassing diet, lifestyle modifications, and environmental factors. As research continues to unveil the complexities of the gut-brain axis, interventions targeting gut health hold promise in ameliorating mental health disorders and overall well-being.
By addressing the various factors that influence gut health, individuals can take proactive steps to support their mental health and improve their quality of life.
Don’t know where to start?
A holistic approach
Dietary Approaches for Gut Health and Mental Well-being:
Incorporate fermented foods like sauerkraut and kefir to promote a healthy gut microbiome.
Increase the consumption of fibre-rich foods such as nuts, seeds, fruits, vegetables, and whole grains to support digestive health.
Consume omega-3 fatty acids daily to reduce inflammation and support brain health. Sources: small fatty fish, chia seeds, and flaxseeds.
Limit intake of processed foods, sugar, and artificial sweeteners, which can disrupt gut bacteria and exacerbate mental health conditions.
Lifestyle Modifications:
Engage in regular physical activity to promote gut motility and reduce stress, which can impact gut health.
Practice stress management techniques such as mindfulness meditation, deep breathing exercises, or yoga to mitigate the effects of stress on the gut-brain axis.
Ensure adequate sleep hygiene by maintaining a consistent sleep schedule and creating a conducive sleep environment, as sleep disturbances can disrupt gut health and mental well-being.
Reduce Exposure to Environmental Factors:
Limit exposure to electromagnetic fields (EMFs) from electronic devices by using protective measures such as shielding materials or reducing screen time before bedtime.
Discuss the appropriate protocol alongside vaccinations with healthcare professionals to minimise potential adverse effects on your gut health and mental well-being.
Filter drinking water with the best filtering system you can afford to remove unwanted toxins, like heavy metals, microplastics, and forever chemicals. If you can only afford an activated charcoal filter, it is fine. It is better than nothing at all.
Reduce your exposure to toxic chemicals, including those in mainstream cleaning products. Learn to make your own. They are cheaper over the long term and much safer.
References:
Adams, PB. Lawson, S. Sanigorski, A. et al. (1996|). Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 31, S157–S161.
Alpert, JE. Fava, M. (1997). Nutrition and depression: The role of folate. Nutrition Reviews. 55, pp. 145–149.
Belkind-Gerson, J. Carreón-Rodríguez, A. Contreras-Ochoa, CO. et al. (2008). Fatty acids and neurodevelopment. Journal of Pediatric Gastroenterology and Nutrition. 47(Suppl 1), S7-S9. doi:10.1097/MPG.0b013e3181818e3f
Benton, D. (2002). Selenium Intake, mood and other aspects of psychological functioning. Nutritional Neuroscience. 5, pp. 363–374.
Benton, D. Haller, J. Fordy, J. (1995). Vitamin supplementation for 1 year improves mood. Neuropsychobiology. 32(2), pp. 98-105. doi:10.1159/000119220
Bourre, JM. (2006). Effect of nutrients (in food) on the structure and function of the nervous system: Update on dietary requirements for brain, Part 1: Micronutrients. The Journal of Nutrition, Health and Aging. 10, pp. 377–385.
Bourre, JM. (2005). Dietary omega-3 Fatty acids and psychiatry: Mood, behavior, stress, depression, dementia and aging. Journal of Nutrition, Health and Aging. 9, pp. 31–38.
Brown, GL. Ebert, MH. Gover, PH. et al. (1982). Aggression, suicide and serotonin: Relationships to CSF amine metabolites. American Journal of Psychiatry. 139, pp. 741–746.
Bruinsma, KA. Taren, DL. (2000). Dieting, essential fatty acid intake, and depression. Nutrition Reviews. 58(4), pp. 98-108. doi:10.1111/j.1753-4887.2000.tb07539.x
Diehl, DJ. Gershon S. (1992). The role of dopamine in mood disorders. Comp Psychiatry. 33, pp. 115–120.
Docherty, J. Sack, DA. Roffman, M. et al. (2005) A double-blind, placebo-controlled exploratory trial of chromium picolinate in atypical depression: Effect on carbohydrate craving. Journal of Psychiatric Practice. 11, pp. 302–314.
Duntas, LH. Mantzou, E. Koutras, EA. (2003). Effects of a six month treatment with selenomethionine in patients with autoimmune thyroiditis. European Journal of Endocrinology. 148, pp. 389–93
Eby, GA. Eby, KL. (2006). Rapid recovery from major depression using magnesium treatment. Medical Hypotheses. 67, pp. 362–370.
Firk, C, Markus, CR. (2007). Serotonin by stress interaction: A susceptibility factor for the development of depression? Journal of Psychopharmacology. 21, pp. 538–544.
Hibbeln, JR. (1998). Fish consumption and major depression. Lancet. 351, 1213.
Hoes, MJ. (1982). L-tryptophan in depression. J Orthomolecular Psychiatry. 4, 231.
Leonard, BE. (1997). The role of noradrenaline in depression: A review. Journal of Psychopharmacology. 11, S39–S47.
Levenson, CW. (2006). Zinc, the new antidepressant? Nutrition Reviews. 6, pp. 39–42.
Marszalek, JR. Lodish, HF. (2005). Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: breastmilk and fish are good for you. Annual Review of Cell and Developmental Biology. 21, pp. 633-657. doi:10.1146/annurev.cellbio.21.122303.120624
McLean, A. Rubinsztein, JS. Robbins, TW. et al. (2004). The effects of tyrosine depletion in normal healthy volunteers: Implications for unipolar depression. Psychopharmacology. 171, pp. 286–297.
Murray, CJL. Lopez, AD. (1996). The global burden of disease. World Health Organization. 1996:270. ISBN: 0-9655466-0-8. Available at: https://iris.who.int/bitstream/handle/10665/41864/0965546608_eng.pdf
National Institute of Mental Health: Depression. National Institute of Mental Health. (2000). (US Department of Health and Human Services, Bethesda (MD) [reprinted September 2002]
Nowak, G. Szewczyk, A. (2005). Zinc and depression, An update. Pharmacological Reports. 57, pp. 713–718.
Petty, F. (1995). GABA and mood disorders: A brief review and hypothesis. Journal of Affective Disorders. 34, pp. 275–281.
Quinn, BP. (1999). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Primary Care Version. Prim Care Companion Journal of Clinical Psychiatry. 1(2), pp. 54–55.
Reis, LC. Hibbeln, JR. (2006). Cultural symbolism of fish and the psychotropic properties of omega-3 fatty acids. Prostaglandins, Leukotrienes & Essential Fatty Acids. 75, 227–236.
Rudin, DO. (1981). The major psychoses and neuroses as omega-3 fatty acid deficiency syndrome: Substrate pellagra. Biological Psychiatry. 16, pp. 837–850.
Ruhe, HG. Mason, NS. Schene, AH. (2007). Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Molecular Psychiatry. 12, pp. 331–59.
Rush, AJ. (2007). The varied clinical presentations of major depressive disorder. Journal of Clinical Psychiatry. 68, p. 4–10.
Shaheen Lakhan, SE. Vieira, KF. (2008). Nutritional therapies for mental disorders. Nutrition Journal. 7:2. doi:10.1186/1475-2891-7-2
Shor-Posner, G. Lecusay, R. Miguez, MJ. et al. (2003. Psychological burden in the era of HAART: Impact of selenium therapy. International Journal of Psychiatry in Medicine. 33(1), pp. 55-69. doi:10.2190/PFFD-D920-V041-N5KD
Sinclair, AJ. Begg, D. Mathai, M. et al. (2007). Omega-3 fatty acids and the brain: Review of studies in depression. Asia Pacific Journal of Clinical Nutrition. 16, pp. 391–397.
Stockmeier, CA. (1997). Neurobiology of serotonin in depression and suicide. Annals of the New York Academy of Sciences. 836, pp. 220–232.
Stoll, AL. Severus, WE. Freeman, MP. et al. (1999). Omega 3 fatty acids in bipolar disorder: A preliminary double-blind, placebo-controlled trial. Archives Of General Psychiatry. 56, pp. 407–412.
Tanskanen, A. Hibbeln, JR. Hintikka, J. et al. (2001). Fish consumption, depression, and suicidality in a general population. Archives Of General Psychiatry. 58, pp. 512–513.
Van Praag, HM. (1983). Depression, suicide and the metabolism of serotonin in the brain. Journal of Affective Disorders. 4, pp. 275–290.
Wells, AS. Read, NW. Laugharne, JD. et al. (1998). Alterations in mood after changing to a low-fat diet. British Journal of Nutrition. 79(1), pp. 23-30. doi:10.1079/bjn19980005
Wurtman, R. O'Rourke, D. Wurtman, JJ. (1989). Nutrient imbalances in depressive disorders: Possible brain mechanisms. Annals of the New York Academy of Sciences. 575, pp. 75–82.
Young, SN. (2007). Folate and depression: A neglected problem. Journal of Psychiatry and Neuroscience. 32, pp. 80–82.