25 Tips to Manage IBS: The Role of HPA Axis Hyperactivation
What is HPA Axis Hyperactivation?
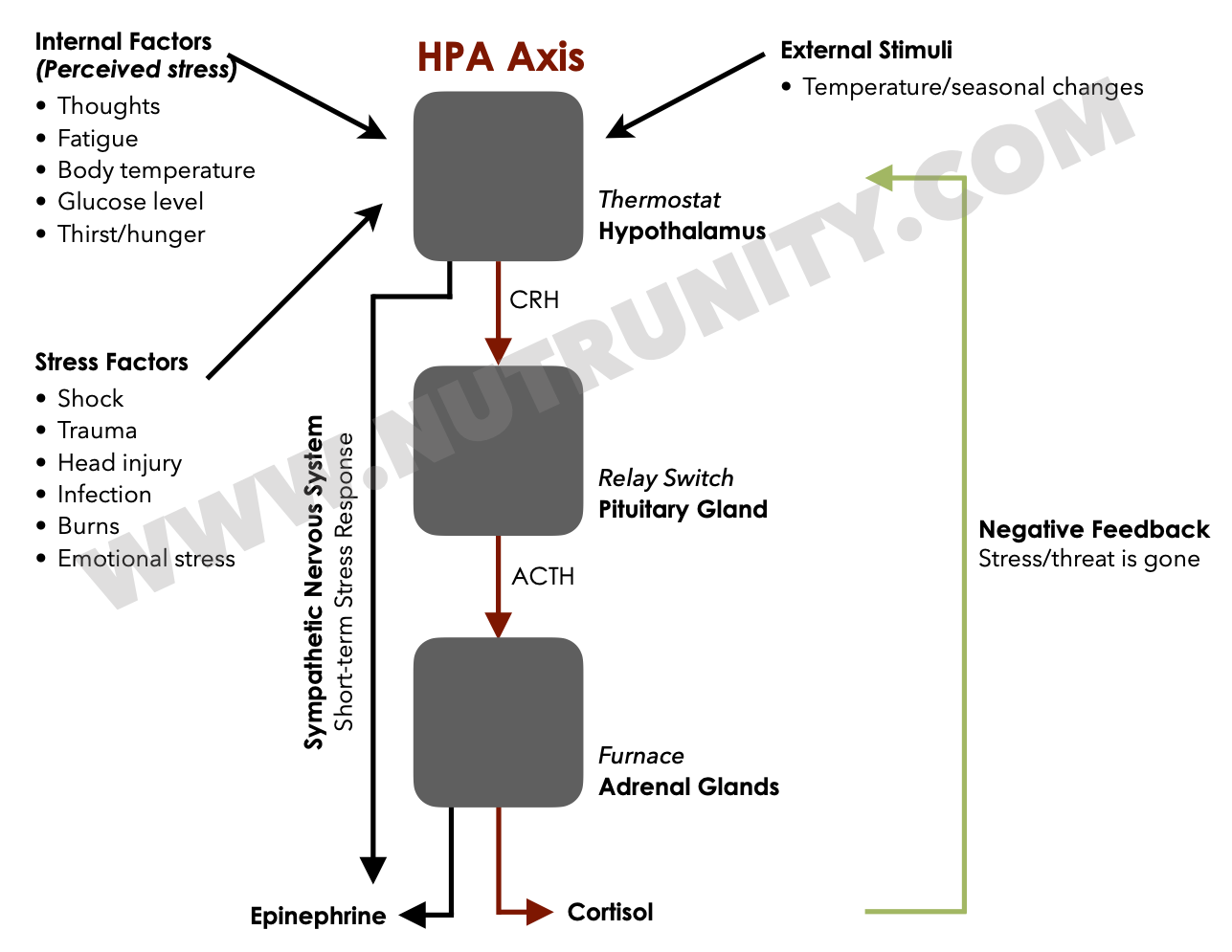
The Hypothalamic-Pituitary-Adrenal (HPA) axis is a complex system of interactions between three key endocrine glands: the hypothalamus, the pituitary gland, and the adrenal glands. The axis regulates the body’s response to stress, digestion, immune system, mood, energy storage, and expenditure. Here’s how it works:
Hypothalamus: When the body perceives stress, the hypothalamus releases corticotropin-releasing hormone (CRH).
Pituitary Gland: CRH stimulates the pituitary gland to release adrenocorticotropic hormone (ACTH).
Adrenal Glands: ACTH then triggers the adrenal glands to produce and release epinephrine, a primary stress hormone. If the stress response is continuously activated, the adrenals then produce cortisol to further assist the body in sequestrating energy and supplying it to the muscle tissue.
Illustration by Olivier Sanchez, extracted from “Energise - 30 Days to Vitality”
HPA Axis Hyperactivation occurs when this system is overstimulated, leading to an excessive production of cortisol and other stress hormones. This can happen due to chronic stress, prolonged exposure to stressful situations, or certain psychological conditions.
Hyperactivation of the HPA axis can lead to various health issues, including:
Immune System Suppression: Excessive cortisol can suppress the immune system, making the body more susceptible to infections.
Digestive Issues: High cortisol levels can affect gut motility and permeability, leading to gastrointestinal problems such as IBS.
Mood Disorders: Chronic stress and elevated cortisol levels are associated with anxiety, depression, and other mood disorders.
Metabolic Changes: Prolonged HPA axis activation can affect metabolism, leading to weight gain, insulin resistance, and other metabolic disorders.
In the context of IBS, HPA axis hyperactivation can exacerbate symptoms by increasing gut sensitivity and altering digestive functions.
Role of the HPA axis
The autonomic nervous system (ANS) is a division of the peripheral nervous system that regulates involuntary bodily functions, ensuring homeostasis and responding to internal and external stimuli. It operates independently of conscious control and maintains vital functions such as heart rate, digestion, respiratory rate, and temperature regulation. The ANS consists of two main branches:
Sympathetic Nervous System (SNS): Activates the body's "fight or flight" response during stress or emergencies, preparing it for action by increasing heart rate, dilating pupils, and redirecting blood flow to muscles.
Parasympathetic Nervous System (PNS): Promotes the body's "rest and digest" response, conserving energy and facilitating bodily functions like digestion, urination, and sexual arousal. It counterbalances the SNS to maintain physiological equilibrium.
The role of the Vagus Nerve
The vagus nerve plays a critical role in the autonomic nervous system (ANS), particularly in the parasympathetic division:
Parasympathetic Regulation: The vagus nerve is a major component of the parasympathetic nervous system (PNS). It innervates many organs, including the heart, lungs, digestive tract, and various glands. Activation of the vagus nerve promotes the "rest and digest" response, facilitating digestion, reducing heart rate, and promoting relaxation.
Influence on Heart Rate: The vagus nerve plays a significant role in regulating heart rate. Stimulation of the vagus nerve slows down the heart rate, whereas reduced vagal activity increases heart rate. This mechanism helps maintain cardiovascular homeostasis.
Digestive Functions: The vagus nerve innervates several organs involved in digestion, including the stomach, pancreas, and intestines. It enhances gastrointestinal motility, stimulates digestive enzyme secretion, and promotes nutrient absorption. Vagal stimulation also triggers the release of gastric acid and bile, essential for digestion.
Respiratory Control: The vagus nerve extends into the lungs and regulates respiratory functions. It influences bronchoconstriction and bronchodilation, regulates airway resistance, and coordinates the respiratory rhythm with other autonomic functions.
Sensory and Reflex Functions: The vagus nerve also carries sensory information from the viscera and the gut to the brainstem. It plays a role in detecting changes in internal organ function and relaying these signals for appropriate autonomic responses.
Role in Inflammation and Immune Response: Recent research indicates that the vagus nerve modulates inflammation and immune responses through the cholinergic anti-inflammatory pathway. Vagal stimulation can suppress inflammatory cytokine release and attenuate excessive immune responses.
The Role of the Sympathetic Nervous System (SNS):
Function: The sympathetic nervous system activates physiological responses that increase alertness, enhance physical performance, and prepare the body to confront or flee danger.
Neurotransmitters: The SNS uses the neurotransmitter norepinephrine (noradrenaline) to transmit signals from nerves to target organs and tissues.
Effects on Organs and Systems:
Heart: Increases heart rate and contractility to pump more blood to muscles and vital organs, and supply essential nutrients (including glucose) to muscle tissue to prepare the body to fight or flight.
Lungs: Dilates airways to increase oxygen intake.
Blood Vessels: Constricts blood vessels in non-essential organs (e.g., digestive system) and dilates vessels in muscles to increase blood flow.
Pupils: Dilates pupils to improve vision.
Adrenal Glands: Stimulates the release of adrenaline (epinephrine) from the adrenal medulla, reinforcing the body's response to stress.
The vagus nerve, its role in “rest-and-digest” and communication with the brain
The parasympathetic nervous system
The parasympathetic nervous system (PNS) works in opposition to the sympathetic nervous system to maintain homeostasis and promote relaxation. Here are some key functions influenced by the parasympathetic system:
Heart Rate: The parasympathetic nervous system slows down heart rate via the vagus nerve, promoting rest and digestion after periods of stress or exertion.
Respiration: It decreases respiratory rate and depth, facilitating relaxation and conserving energy.
Digestion: The parasympathetic system stimulates digestion by increasing saliva production, promoting peristalsis (movement of food through the digestive tract), and enhancing the secretion of digestive enzymes and bile.
Urination: It promotes bladder contraction and relaxation of the urinary sphincters, facilitating urination.
Defecation: It stimulates smooth muscle contraction in the intestines, aiding in waste elimination.
Sexual Arousal: The parasympathetic system plays a role in sexual arousal by promoting genital blood flow and lubrication.
Pupillary Constriction: It causes constriction of the pupil (miosis), which enhances near vision and protects the eye from excessive light.
Salivation: It increases saliva production, aiding in digestion and maintaining oral health.
Gastric Acid Secretion: It stimulates gastric acid secretion, which helps break down food.
Immune Response: The parasympathetic system may also modulate immune function, promoting anti-inflammatory responses.
Overall, the parasympathetic nervous system is essential for conserving energy, promoting digestion and elimination, facilitating relaxation, and supporting sexual arousal and other restorative processes in the body. Its actions counterbalance those of the sympathetic nervous system to maintain physiological equilibrium and adapt to changing internal and external environments.
Irritable Bowel Syndrome (IBS) is a prevalent gastrointestinal disorder that affects millions of people globally. It is characterised by chronic abdominal pain, discomfort, bloating, and altered bowel habits, which can significantly impact daily life.
Despite its widespread prevalence, IBS remains a misunderstood condition, because doctors are often unable to pinpoint the problem and link it to a drug (to suppress the symptoms, while ignoring the cause).
This article aims to provide an in-depth understanding of IBS, exploring its possible causes, symptoms, and a comprehensive list of solutions to alleviate and manage symptoms effectively.
Possible Causes of IBS
IBS is a multifaceted condition with a variety of potential causes. Understanding these underlying factors can help in managing and alleviating symptoms.
— Gut-Brain Axis Dysregulation
The gut-brain axis is a complex communication network that links the gastrointestinal tract with the brain. This bidirectional communication supports gut health and overall well-being. Dysregulation of this axis, often due to stress or psychological factors, can lead to the onset of IBS symptoms. For instance, stress and anxiety can trigger the release of neurotransmitters that affect gut motility and sensitivity, leading to symptoms like abdominal pain and altered bowel habits.
— Stress and HPA Axis Hyperactivation
Stress is a significant factor in IBS, with many patients reporting symptom flare-ups during stressful periods. The Hypothalamic-Pituitary-Adrenal (HPA) axis is responsible for the body’s stress response. Chronic stress can lead to hyperactivation of the HPA axis, resulting in the overproduction of cortisol and other stress hormones. This hormonal imbalance can affect gut motility, increase gut permeability, and alter gut microbiota, exacerbating IBS symptoms.
— Altered Gut Microbiota
The gut microbiota, composed of trillions of microorganisms, is key to digestive health, metabolism and immune function. An imbalance in these microorganisms, known as dysbiosis, can contribute to IBS. Factors such as antibiotics, poor diet, and stress can disrupt the balance of gut bacteria, leading to symptoms like bloating, gas, and altered bowel habits.
— Food Sensitivities and Intolerances
Certain foods can trigger IBS symptoms in susceptible individuals. Common triggers include dairy products, gluten, and high-FODMAP foods (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols). These foods are poorly absorbed in the small intestine, leading to fermentation by gut bacteria and resulting in gas, bloating, and discomfort.
Following a low-FODMAP diet typically involves three phases:
Elimination Phase (6-8 weeks): During this period, high-FODMAP foods are strictly avoided to see if symptoms improve.
Reintroduction Phase (6-8 weeks): Gradually reintroduce high-FODMAP foods one at a time to identify which ones trigger symptoms.
Personalisation Phase (ongoing): Develop a long-term eating plan that includes only tolerable amounts of high-FODMAP foods.
DO NOT EXCEED 8 WEEKS ON A LOW FODMAP DIET!
— Hormonal Imbalances
Hormonal changes, particularly in women, can influence IBS symptoms. Many women report worsening symptoms during menstruation, pregnancy, or menopause, suggesting that hormonal fluctuations play a role in IBS. Oestrogen and progesterone, in particular, can affect gut motility and sensitivity.
— Genetic Factors
Genetics can also play a role in the development of IBS. A family history of IBS increases the likelihood of developing the condition, indicating a potential genetic predisposition. Studies have identified certain genetic variations that may influence gut motility, immune function, and pain sensitivity, contributing to IBS.
— Increased Intestinal Permeability
Increased intestinal permeability, commonly known as “leaky gut syndrome,” can allow harmful substances to pass through the gut lining into the bloodstream. This can trigger an immune response, leading to inflammation and IBS symptoms. Factors such as stress, poor diet, food emulsifiers and other additives widely found in ultra-processed junk, and infections can increase gut permeability.
— Neurotransmitter Imbalances
Neurotransmitters like serotonin play a crucial role in gut motility and sensitivity. An imbalance in serotonin levels can affect bowel function, leading to symptoms like diarrhoea, constipation, and abdominal pain. Up to 90% of the serotonin in circulation is produced in the gut, highlighting the importance of gut health in managing IBS.
— Increased Glutamate Levels
Glutamate is an excitatory neurotransmitter involved in various brain functions, including learning and memory. In the context of IBS, glutamate plays a critical role in pain perception and the functioning of the central nervous system. Elevated levels of glutamate can lead to increased excitatory signals in the gut-brain axis, contributing to heightened pain sensitivity, a hallmark of IBS known as visceral hypersensitivity. This heightened sensitivity makes normal intestinal activities feel painful and uncomfortable.
The overactivation of glutamate receptors in the gut can also lead to increased motility and secretion, exacerbating symptoms like diarrhoea. Moreover, glutamate can influence the release of other neurotransmitters and inflammatory mediators, further aggravating gut inflammation and discomfort. Managing glutamate levels and modulating its activity through dietary and medical interventions can help alleviate these symptoms.
— Post-Infectious Changes
IBS can develop after a severe gastrointestinal infection, a condition known as post-infectious IBS. The infection can cause long-lasting changes in the gut, including altered gut motility, increased gut permeability, and changes in gut microbiota, contributing to IBS symptoms and other conditions such as SIBO, and together contribute to a wide range of symptoms. It may also be linked to SIYO or candidiasis, leading to the development of candida in the gut and disturbing other microbiomes, such as the vaginal microbiota.
— Small Intestinal Bacterial Overgrowth (SIBO), Small Intestinal Yeast Overgrowth (SIYO), and Small Intestinal Fungal Overgrowth (SIFO)
SIBO, SIYO, and SIFO are conditions that can overlap with IBS, characterised by an abnormal increase in the number of bacteria, yeast, or fungi in the small intestine (where they should not be in such numbers). These overgrowths can result from altered gut motility and gut inflammation, leading to symptoms like bloating, gas, diarrhoea, and abdominal pain.
SIBO: This occurs when bacteria that usually reside in the large intestine migrate to the small intestine, causing fermentation of food, gas production, and inflammation. Very often, good gut bacteria are involved, producing excessive gas as they start digesting the food you eat and “stealing” vital nutrients.
SIYO: Similar to SIBO, but involves an overgrowth of yeast such as Candida, which can produce toxic byproducts and lead to inflammation and nutrient malabsorption, metabolic dysfunction and chronic issues, such as fatigue and brain fog (poor energy management).
SIFO: Involves an overgrowth of fungi in the small intestine, contributing to similar symptoms as SIBO and SIYO. Saccharomyces cerevisiae: Also known as brewer's yeast, Saccharomyces cerevisiae can colonise the gut and potentially contribute to SIFO when its growth is uncontrolled. It is commonly used in food fermentation processes and as a probiotic but can overgrow in the gut in certain individuals. Other fungal species: Besides Candida and Saccharomyces, Aspergillus and Cryptococcus have been reported to contribute to fungal overgrowth in the small intestine in some cases. These fungi may enter the gut through contaminated food or water.
— Symptoms of IBS
IBS presents a range of symptoms that can vary in severity and frequency among individuals. Common symptoms include:
Abdominal Pain and Cramping: Often relieved by bowel movements.
Bloating and Gas: Excess gas production leading to discomfort and bloating.
Diarrhoea or Constipation: Some individuals experience diarrhoea (IBS-D), others constipation (IBS-C), and some alternating between the two (IBS-M).
Mucus in Stool.
Fatigue: Chronic fatigue and low energy levels.
Difficulty Sleeping: Poor sleep quality and disturbances.
Anxiety and Depression: Psychological symptoms often accompany IBS.
25 Solutions and Tips to Alleviate IBS Symptoms
Managing IBS involves a holistic approach that includes dietary adjustments, lifestyle modifications, and the combination of different therapies.
Here are 25 solutions and tips to help alleviate and manage IBS symptoms effectively.
Dietary Adjustments
Follow a Low-FODMAP Diet
A low-FODMAP diet is designed to reduce fermentable sugars that can cause gas and bloating. Foods like onions, garlic, beans, and certain fruits and vegetables are high in FODMAPs and should be limited. The elimination phase lasts 6-8 weeks, followed by a reintroduction phase of the same duration, and finally a personalisation phase for long-term management.
Stay Hydrated
Drinking plenty of water helps with digestion and bowel movements. Aim for at least 8 cups of water a day to keep your digestive system functioning smoothly.
Eat Smaller, Frequent Meals
Large meals can overwhelm the digestive system. Eating smaller, more frequent meals can help reduce bloating and discomfort.
Incorporate Fiber Gradually
Soluble fibre in foods like oats, bananas, flax/linseeds and sweet potatoes, can help prevent constipation. However, introduce it gradually to avoid gas and bloating.
Avoid Trigger Foods
Identify and eliminate foods that trigger your symptoms. Common triggers include dairy, gluten, fatty foods, certain emulsifiers and artificial sweeteners. Identify food allergies that may also contribute to the problem.
Lifestyle Modifications
Regular Physical Activity
Exercise helps reduce stress and improves bowel function. Aim for at least 30 minutes of moderate exercise, like walking or cycling, most days of the week.
Practice Stress-Relief Techniques
Yoga, meditation, and deep-breathing exercises can help manage stress. Stress management is crucial for reducing IBS symptoms.
Get Adequate Sleep
Quality sleep is essential for overall health and can reduce IBS symptoms. Aim for 7-9 hours of sleep per night and establish a regular sleep routine.
Limit Caffeine and Alcohol
Both can irritate the digestive system and worsen IBS symptoms. Try to limit or avoid caffeine and alcohol to see if your symptoms improve.
Quit Smoking
Smoking can worsen IBS symptoms. If you smoke, seek help to quit and improve your overall health and digestive function.
Medical and Alternative Treatments
Probiotics
Probiotic supplements can help balance gut bacteria and improve IBS symptoms. Look for strains like Bifidobacterium and Lactobacillus, which are known to benefit gut health. However, do not use probiotics if you suspect SIBO/SIYO/SIFO.
Peppermint Oil
Peppermint oil capsules can relieve IBS symptoms by relaxing intestinal muscles and reducing pain and bloating. Enteric-coated capsules are recommended to prevent heartburn.
Antispasmodic Medications
These medications can reduce cramping and pain. Your doctor may prescribe those to reduce the severity of your symptoms.
Cognitive Behavioral Therapy (CBT)
CBT can help manage stress and change patterns of thinking that may contribute to IBS. Therapy can be done in person or through online programs.
Acupuncture
Some individuals find relief through acupuncture, which can reduce pain and stress. Ensure you see a licensed and experienced practitioner.
Supplements and Herbal Remedies
Psyllium Husk
Containing soluble fibre, psyllium husk can help with constipation. Start with a small dose and gradually increase to avoid gas and bloating. Drink plenty of water as it will draw a large amount of liquid from your tissue.
Ginger
Ginger can reduce nausea and improve digestion. Fresh ginger tea or liquidised ginger (about the size of 1 shot, 25 ml) can be beneficial.
Magnesium Supplements
Magnesium can help relax the muscles in the gut and improve bowel movements. Magnesium citrate is often recommended for constipation (do not exceed dosage as it will lead to diarrhoea).
L-Glutamine
An amino acid that can help with intestinal permeability and inflammation. It is available as a powder that can be mixed with water or juice. Bone broth is also packed with glutamine. Be careful to not overdo it as glutamine is converted to glutamate (especially when you are under stress) and can participate in the increased feeling of pain.
Turmeric
Turmeric has anti-inflammatory properties that can help with IBS. Curcumin, the active ingredient, can be taken as a supplement, tea or concoction with ginger and coconut milk (golden milk).
Behavioural and Cognitive Strategies
Mindfulness-based stress Reduction (MBSR)
MBSR can help reduce stress and improve symptoms. It involves mindfulness meditation and body awareness practices.
Biofeedback Therapy
This technique helps you learn to control physiological functions like heart rate and muscle tension, which can reduce stress and IBS symptoms.
Hypnotherapy
Gut-directed hypnotherapy can reduce pain and other IBS symptoms. It involves guided relaxation and focused attention techniques.
Journaling
Keeping a symptom diary can help identify triggers and manage stress. Track your symptoms, diet, and stress levels to find patterns. It is also an opportunity to write down triggers that can affect your emotions and become more mindful and in control (use intentions to organise your day and affirmations to control your emotions).
Join a Support Group
Connecting with others who have IBS can provide emotional support and practical advice. Support groups can be found online or in your local community.
Conclusion
IBS is a complex condition with a variety of potential causes and symptoms. By understanding these factors and implementing the solutions provided, individuals can manage their symptoms more effectively. Whether through dietary changes, lifestyle adjustments, or medical treatments, there are numerous ways to alleviate the discomfort and improve the quality of life for those living with IBS.
By addressing the gut-brain connection, reducing stress, and making informed lifestyle choices, managing IBS becomes a more achievable goal.
References:
Brenner, DM. Moeller, MJ. Chey, WD. et al. (2009). The utility of probiotics in the treatment of irritable bowel syndrome: A systematic review. American Journal of Gastroenterology. 104(4), pp. 1033-1049; quiz 1050. doi:10.1038/ajg.2009.25
Dickson, K. Malitan, H. Lehmann, C. (2020). Imaging of the intestinal microcirculation during acute and chronic inflammation. Biology (Basel). 9(12), 418. doi:10.3390/biology9120418
Furgała, A. Mazur, M. Jabłoński, K. et al. (2008). Myoelectric and autonomic nervous system activity in patients with irritable bowel syndrome. Folia medica Cracoviensia. 49(3-4), pp. 49-58.
Groeger, D. Murphy, EF. Tan, HTT. et al. (2023). Interactions between symptoms and psychological status in irritable bowel syndrome: An exploratory study of the impact of a probiotic combination. Neurogastroenterology & Motility. 35(1), e14477. doi:10.1111/nmo.14477
Hu, C. Yan, C. Wu, Y. et al. (2023). Low FODMAP Diet relieves visceral hypersensitivity and is associated with changes in colonic microcirculation in water avoidance mice model. Nutrients. 15(5), 1155. doi:10.3390/nu15051155
Katherine Jurek, M. Seavey, H. Guidry, M. et al. (2022). The effects of slow deep breathing on microvascular and autonomic function and symptoms in adults with irritable bowel syndrome: A pilot study. Neurogastroenterology & Motility. 34(5), e14275. doi:10.1111/nmo.14275
Lewis, ED. Antony, JM. Crowley, DC. et al. (2020). Efficacy of Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in alleviating symptoms of irritable bowel syndrome (IBS): A randomized, placebo-controlled study. Nutrients. 12(4), 1159. doi:10.3390/nu12041159
Liu, J. Chey, WD. Haller, E. et al. (2020). Low-FODMAP Diet for Irritable Bowel Syndrome: What We Know and What We Have Yet to Learn. Annual Review of Medicine. 71, pp. 303-314. doi:10.1146/annurev-med-050218-013625
Liu, J. Lv, C. Wang, W. et al. (2022). Slow, deep breathing intervention improved symptoms and altered rectal sensitivity in patients with constipation-predominant irritable bowel syndrome. Frontiers in Neuroscience. 16, 1034547. doi:10.3389/fnins.2022.1034547
Manabe, N. Tanaka, T. Hata, J. et al. (2009). Pathophysiology underlying irritable bowel syndrome--from the viewpoint of dysfunction of autonomic nervous system activity. Journal of Smooth Muscle Research. 45(1), pp. 15-23. doi:10.1540/jsmr.45.15
Mansueto, P. Seidita, A. D'Alcamo, A. et al. (2015). Role of FODMAPs in patients with irritable bowel syndrome. Nutrition in Clinical Practice. 30(5), pp. 665-682. doi:10.1177/0884533615569886
Martínez-Martínez, LA. Mora, T. Vargas, A. et al. (2014). Sympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: A review of case-control studies. Journal of Clinical Rheumatology. 20(3), pp. 146-150. doi:10.1097/RHU.0000000000000089
Morariu, ID. Avasilcai, L. Vieriu, M. et al. (2023). Effects of a low-FODMAP diet on irritable bowel syndrome in both children and adults — A narrative review. Nutrients. 15(10), 2295. doi:10.3390/nu15102295
Ohman, L. Simrén, M. (2007). New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Digestive and Liver Disease. 39(3), pp. 201-215. doi:10.1016/j.dld.2006.10.014
Pinto-Sanchez, MI. Hall, GB. Ghajar, K. et al. (2017). Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology. 153(2), pp. 448-459.e8. doi:10.1053/j.gastro.2017.05.003
Shi, X. Hu, Y. Zhang, B. et al. (2021). Ameliorating effects and mechanisms of transcutaneous auricular vagal nerve stimulation on abdominal pain and constipation. JCI Insight. 6(14), e150052. doi:10.1172/jci.insight.150052
So, D. Loughman, A. Staudacher, HM. (2022). Effects of a low FODMAP diet on the colonic microbiome in irritable bowel syndrome: A systematic review with meta-analysis. American Journal of Clinical Nutrition. 116(4), pp. 943-952. doi: 10.1093/ajcn/nqac176
Spaziani, R. Bayati, A. Redmond, K. et al. (2008). Vagal dysfunction in irritable bowel syndrome assessed by rectal distension and baroreceptor sensitivity. Neurogastroenterology & Motility. 20(4), pp. 336-342. doi:10.1111/j.1365-2982.2007.01042.x
Staudacher, HM. Whelan, K. (2016). Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: Probiotics, prebiotics and the low FODMAP diet. Proceedings of the Nutrition Society. 75(3), pp. 306-318. doi:10.1017/S0029665116000021
Tanaka, T. Manabe, N. Hata, J. et al. (2008). Characterization of autonomic dysfunction in patients with irritable bowel syndrome using fingertip blood flow. Neurogastroenterology & Motility. 20(5), pp. 498-504. doi:10.1111/j.1365-2982.2007.01039.x
Tillisch, K. Mayer, EA. Labus, JS. et al. (2005). Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 54(10), pp. 1396-1401. doi:10.1136/gut.2004.058685
Varjú, P. Farkas, N. Hegyi, P. et al. (2017). Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: A meta-analysis of clinical studies. PLoS One. 12(8), e0182942. doi:10.1371/journal.pone.0182942
Weynants, A. Goossens, L. Genetello, M. et al. (2020). The long-term effect and adherence of a low fermentable oligosaccharides disaccharides monosaccharides and polyols (FODMAP) diet in patients with irritable bowel syndrome. Journal of Human Nutrition and Dietetics. 33(2), pp. 159-169. doi:10.1111/jhn.12706
Wong, WM. (2016). Restriction of FODMAP in the management of bloating in irritable bowel syndrome. Singapore Medical Journal. 57(9), pp. 476-484. doi:10.11622/smedj.2016152
Yuan, F. Ni, H. Asche, CV. et al. (2017). Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: A meta-analysis. Current Medical Research and Opinion. 33(7), pp. 1191-1197. doi:10.1080/03007995.2017.1292230